ATORVASTATIN
Product Details:
- HS Code 29420090
- Molecular Weight 558.64 g/mol GSM (gm/2)
- Shelf Life 3 Years
- Molecular Formula C33H35FN2O5
- Storage Room Temperature
- Medicine Name Atrovastatin
- Chemical Name Atrovastatin
- Click to View more
ATORVASTATIN Price And Quantity
- 5 Kilograms
- 18500 INR/Kilograms
ATORVASTATIN Product Specifications
- 3 Years
- 558.64 g/mol GSM (gm/2)
- off-white to white powder
- Powder
- Atorvastatin is one type of statin medication which is used to prevent the cardio vascular disease. Atorvastatin is a first line medication which is used in treatment of cardio vascular disease. Atorvastatin belongs to the HMG-CoA reductase inhibitors class of medicine.
- C33H35FN2O5
- Other
- Medicine Grade
- Room Temperature
- 29420090
- 99 %
- Atrovastatin
- Atrovastatin
- 134523-00-5
ATORVASTATIN Trade Information
- SAHAR AIR CARGO
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Dadra and Nagar Haveli Daman and Diu Arunachal Pradesh Uttarakhand Gujarat Tamil Nadu Central India Odisha Nagaland North India West Bengal Delhi Punjab Bihar West India Uttar Pradesh Chandigarh South India Andaman and Nicobar Islands Goa Sikkim Jammu and Kashmir Andhra Pradesh Jharkhand Chhattisgarh Pondicherry Haryana Himachal Pradesh Kerala Manipur Karnataka Lakshadweep East India Assam Telangana Meghalaya Tripura Rajasthan Madhya Pradesh Mizoram Maharashtra All India
- FDCA, GMP, GLP AND ISO
Product Description
Niksan Pharmaceutical is among worlds top most manufacturer, supplier, exporter and trader of the Atorvastatin finished formulations moreover Atorvastatin API. Niksan Pharmaceutical is the huge manufacturer, exporter and supplier of Atorvastatin API and formulation situated in Ankleshwar, Gujarat, India.
Niksan Pharmaceutical is the manufaturer, exporter and supplier of Atorvastati n API and Atorvastatin in all over the world.
Our product Atorvastatin is world widely appreciated by our group companies and clients. We give best possible selling price of Atorvastatin to our clients, because customer satisfaction is our top priority.
Niksan Pharmaceutical exporting the Atorvastatin API, Intermediates and finished formulation internationally from many years in countries like Puerto Rico, United States, Philippines, New Zealand, SriLanka, Canada, United Kingdom, Finland, Ireland, Jordan, Indonesia, Singapore, Sweden, Germany, Australia, Switzerland, Vietnam, Iraq, United Arab Emirates, Bangladesh,Denmark, Malaysia, Kenya, Austria, Norway, Nigeria, Hungary, Hong Kong, Saudi Arabia, Pakistan, Slovakia, Thailand, South Korea, Argentina, South Africa,Iran, Czechia, Israel, Taiwan, Greece, Egypt Romania, Netherlands, Turkey,Poland, Mexico, Spain, France, Italy, Brazil, Japan and many other countries.
Niksan Pharmaceutical also supply the Atorvastatin API, Intermediates and finished formulations in all Indian states like Gujarat,Mahrashtra, Punjab, Delhi, Tamilnadu, Goa, Uttar Pradesh, Karnataka, Jammu & Kashmir, West Bengal, Assam, Rajasthan, and Hyderabad Etc.
Atorvastatin is one type of statin medication which is used to prevent the cardio vascular disease. Atorvastatin is a firstline medication which is used in treatment of cardio vascular disease. Atorvastatin belongs to the HMG-CoA reductase inhibitors class of medicine.
SYNONYMS: Atorvastatin,atorvastatina, atorvastatine, atorvastatinum.
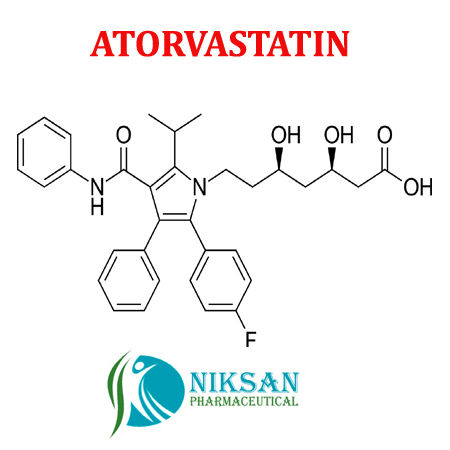
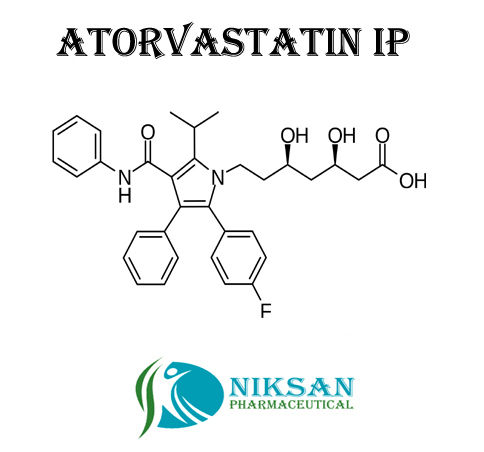
IUPAC NAME OF ATORVASTATIN: (3R, 5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoicacid
CAS NO: 134523-00-5
MOLECULAR FORMULA: C33H35FN2O5
MOLECULAR MASS: 558.64g/mol
STORAGE CONDITION: Store the medication in its original container.Store it in a dry place at a room temperature,away from light and moisture. Do not put medication in bathroom, fridge or in kitchen. Keep it away of reach of children and pet.
HOW TO USE ATORVASTATIN: Take the medication by mouth with or without food one time per day. In case of any confusio ask your doctor for advice.Take medication regularly for the better result, do not miss the dose.
HOW ATORVASTATIN WORKS: Atorvastatin inhibits the production of cholesterol in the body, by this the amount of Cholesterol will decreased. Because of the high cholesterol, it will build up walls of fat in the arteries and blocks the flow of blood that cause high blood pressure.
PHARMACOKINETICS OF ATORVASTATIN: Atorvastatin will rapidly absorb after the oral administration. Atorvastatin reach the peak plasma concentration in about 1-2 hrs.The normal volume of distribution of Atorvastatin is likely 380litre. The half-life of Atorvastatin is about 14 hrs and the metabolites of Atorvastatin can reach up to 30hrs.Atorvastatin is primary excreted by biliary excretion. Very less amount like 2% is recovered by the urination.
SIDE EFFECTS: There are some effects like memory loss and problem in remembering is common. The other effects like confusion, weakness, muscle pain, and kidney problems if these type of effects seen contact your doctor. Atorvastatin can worsen the diabetes, so kindly talk to your doctor about the risks. There are other allergic reactions like rash,itching, swelling can also see in some patients.
PRECAUTIONS: Atorvastatin medication must not be taken in the pregnancy because it can harm the baby. Kindly tell your doctor about your past disease like kidney, heart problem, and liver problem lung problem before writing the prescription.taking alcohol along with this medicine can cause liver problem, so kindly avoid taking alcohol.
CDSCO APPROVAL: S (-) Metoprolol 25mg/50mg (ER) +Atorvastatin (IR) 10mg tablets approved by CDSCO in India in 01.04.2010,
Atorvastatin 10mg +Ramipril 5mg + Aspirin (EC pellets) 75mg/150mg + Metoprolol (ER tablets) 25mg Capsules approved by CDSCO in India in 05.04.2010,
Atorvastatin (40mg) +Ezetimibe (10mg) Tablet (addl. Strength) approved by CDSCO in India in 30.07.2007,
Atorvastatin + Fenofibrate approved by CDSCO in India in 24.12.2004,
Telmisartan 40mg + Atorvastatin (as calcium) 10mg Tablets approved by CDSCO in India in 22.05.2008,
Atorvastatin calcium (amorphous) approved by CDSCO in India in 27.05.2005,
Atorvastatin Calcium (10mg)+ Ramipril (2.5mg/5mg) tablet approved by CDSCO in India in 15.10.2007,
Atorvastatin 5mg + Fenofibrate 160mg (additional strength)approved by CDSCO in India in 01.12.2006,
Atorvastatin Calcium (10mg) + Fenofibrate (145mg) (addl. strength)approved by CDSCO in India in 30.10.2007,
FDC of atorvastatin +fenofibrate approved by CDSCO in India in 01.08.2006,
Atorvastatin 10mg + Aspirin75/150mg Capsules approved by CDSCO in India in 18.01.2008,
Olmesartan (20/40mg) + Atorvastatin approved by CDSCO in India in 27.10.2009
Glimepiride 1/2 mg + Metformin 500 mg + Atorvastatin 10 mg tablets approved by CDSCO in India in 17.12.2009,
Atorvastatin +Metformin(10mg + 500mg SR) Tablets approved by CDSCO in India in 04.12.2008,
Atorvastatin 10mg + Fenofibrate 80mg tablet (Additional Indication) approved by CDSCO in India in 25.01.08,
Atorvastatin calciumtablets approved by CDSCO in India in 17-09-1999,
Metoprolol Succinate Equivalent to Metoprolol Tartrate [ER25/50mg] + Atorvastatin (As calcium) 10mg tablets approved by CDSCO in India in28.01.08,
Atorvastatin + Ezetimibe tabs approved by CDSCO in India in 13/08/2004,
Atorvastatin calcium 5mg/10mg/20mg/40mg/80mg (Additional Dosage Form) approved by CDSCO in India in 20.04.11,
FDC of Atorvastatin (10mg)+ Niacin (375mg/500mg E.R) Capsule approved by CDSCO in India in 26/07/2005,
Amlodipine besylate +Atorvastatin tablets approved by CDSCO in India in 11/02/2004,
Atorvastatin Calcium 80mg tablets (Additional Strength) approved by CDSCO in India in 25.02.08,
S(-) amlodipine + Atorvastatin approved by CDSCO in India in 10/02/2004,
Atorvastatin approved by CDSCO in India in 30/10/2001,
Atorvastatin 20mg + Ramipril2.5mg/5mg Capsules approved by CDSCO in India in 17.03.10.
FORMULATIONS AVAILABLE IN MARKET:
Atorvastatin 5mg Tablets
Atorvastatin 10mg Tablets
Atorvastatin 20mg Tablets
Atorvastatin 30mg Tablets
Atorvastatin40mg Tablets
Atorvastatin 80 mg Tablets
Atorvastatin Calcium (10mg) + Ramipril (2.5mg/5mg) tablets
S(-)Metoprolol 25mg/50mg (ER) + Atorvastatin (IR)10mg tablets
Atorvastatin 10mg + Ramipril 5mg + Aspirin (EC pellets) 75mg/150mg+ Metoprolol (ER tablets) 25mg Capsules
Atorvastatin + Fenofibrate
Telmisartan 40mg + Atorvastatin (as calcium) 10mg Tablets
Atorvastatin calcium (amorphous)
Atorvastatin 5mg + Fenofibrate 160mg (additional strength)
Atorvastatin Calcium (10mg) + Fenofibrate (145mg) (addl. strength)
Olmesartan (20/40mg) + Atorvastatin tablets
Atorvastatin + Metformin (10mg + 500mg SR) Tablets
FDC of Atorvastatin (10mg) + Niacin (375mg/500mg E.R) Capsule
Amlodipine besylate + Atorvastatin tablets
Atorvastatin Calcium 80mg tablets (Additional Strength
Atorvastatin 20mg + Ramipril 2.5mg/5mg Capsules
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese