DOMPERIDONE
Product Details:
- Storage Room Temperature
- Molecular Formula C22H24ClN5O2
- HS Code 29420090
- Shelf Life 3 Years
- Molecular Weight 425.91 g/mol Grams (g)
- Medicine Name DOMPERIDONE
- Chemical Name DOMPERIDONE
- Click to View more
DOMPERIDONE Price And Quantity
- 5 Kilograms
- 2900 INR/Kilograms
- 2900.00 - 4200.00 INR/Kilograms
DOMPERIDONE Product Specifications
- DOMPERIDONE
- Other
- Room Temperature
- 57808-66-9
- Domperidone belongs to the D2 receptor antagonist class of medicine. Domperidone mostly used as an antiemetic agent. It is also used as galactagogue. The drug also used to prevent the nausea and vomiting.
- C22H24ClN5O2
- Powder
- White to Off-White Solid
- 29420090
- 3 Years
- 425.91 g/mol Grams (g)
- DOMPERIDONE
- Medicine Grade
- 99 %
DOMPERIDONE Trade Information
- NHAVA SHEVA
- Cash Against Delivery (CAD) Cash on Delivery (COD) Letter of Credit (L/C) Western Union Paypal Letter of Credit at Sight (Sight L/C) Cash Advance (CA) Telegraphic Transfer (T/T) Delivery Point (DP) Days after Acceptance (DA) Cash in Advance (CID) Cheque
- 100 Kilograms Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Tripura Manipur Himachal Pradesh Andaman and Nicobar Islands Pondicherry Nagaland Uttarakhand Daman and Diu Dadra and Nagar Haveli Lakshadweep South India Central India North India East India West India Andhra Pradesh Assam Arunachal Pradesh Bihar Chandigarh Delhi Goa Jammu and Kashmir Jharkhand Madhya Pradesh Maharashtra Mizoram Meghalaya Punjab Rajasthan Sikkim Tamil Nadu Telangana West Bengal Kerala Haryana Gujarat Uttar Pradesh Karnataka Odisha Chhattisgarh All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceutical is worlds leading manufacturer, trader, exporter and supplier of the Domperidone API and Domperidone formulations. Niksan Pharmaceutical manufactures large quantity of Domperidone API and finished products in Ankleshwar, Gujarat, India. Niksan Pharmaceutical and Niksan group companies are the largest manufacturers and suppliers of Domperidone products.
Niksan Pharmaceutical exporting very big quantity of the fines quality products of Domperidone in all over world for many years in a countries like Sri Lanka, Myanmar (Burma), Nepal, Indonesia,Philippines, Singapore, Canada, Bangladesh, Cambodia, New Zealand, Oman, Qatar,Kenya, United Arab Emirates, Pakistan, Sudan, Thailand, Ghana, Malaysia, ,Iraq, Jordan, France, Hong Kong, Lebanon , United Kingdom, Saudi Arabia,Algeria, Belgium, Australia, Ireland, Kuwait, Italy, Taiwan, Nigeria, Vietnam,Iran, Egypt, United States, Morocco, South Africa, South Korea, Switzerland,Venezuela, Greece, Netherlands, Sweden, Mexico, Spain, Germany, Turkey, Brazil,Japan and many more countries.
Niksan Pharmaceutical also supplies large quantity of Domperidone products in Indian states like Gujarat, Haryana, Rajasthan, Madhya Pradesh, Bihar, Uttar Pradesh, Assam,Goa, Hyderabad, Telangana, Kerala, Tamilnadu, Delhi, Mizoram, Sikkim etc.
Domperidone belongs to the D2 receptor antagonist class of medicine. Domperidone mostly used as an antiemetic agent. It is also used as galactagogue. The drug also used to prevent the nausea and vomiting.
SYNONYMS: Omperidona, Domperidone, Domperidonum.
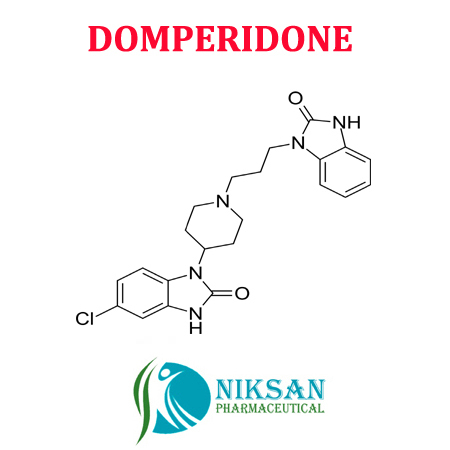
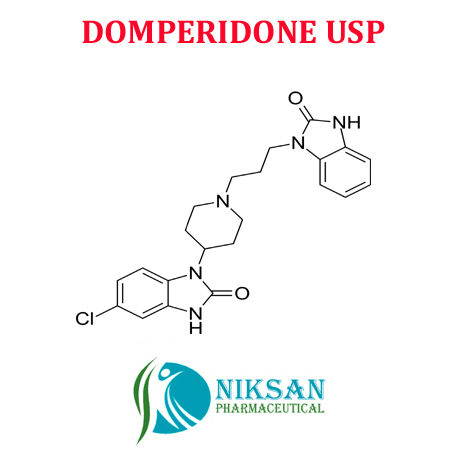
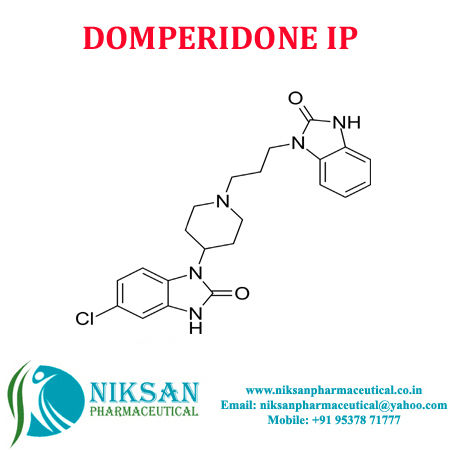
IUPAC NAME: 5-chloro-1-{1-[3-(2-oxo-2, 3-dihydro-1H-1,3-benzodiazol-1-yl)propyl]piperidin-4-yl}-2,3-dihydro-1H-1,3-benzodiazol-2-one
CAS NO: 57808-66-9
FORMULA: C22H24ClN5O2
MOLECULAR MASS: 425.91g/mol
STORAGE CONDITION: Store in room temperature in dry place. Keep away from direct sun light and do not put in bathroom. Keep away from children and pet.
HOW TO TAKE: Take the medicine by mouth 30 min before taking the meals and at the bed time. Do not take over dose this may cause side effects. Take your doctors advice if you have any confusion about taking of dose.
HOW DOMPERIDONE WORKS:Domperidone is the blocker of dopamine receptor. Domperidone blocks the dopamine receptor by this Domperidone cause pro lactin release in the body tha tinhibits the nausea and vomiting. Domperidone also delays the GI emptying.
PHARMACOKINETICS OF DOMPERIDONE: Domperidone absorbed rapidly in the body after the oral administration. Domperidone reaches the peak plasma concentration in 1hr after the dosing. Domperidone binds with approximately 91-93% of plasma protein.The half life of Domperidone is 7 hours. The Domperidone takes time to eliminate from body;the 40mg dose of Domperidone takes 4 days to eliminate 31% of the metabolites.
SIDE EFFECTS OF DOMPERIDONE: The common side effects of Domperidone are irritability, confusion, dry mouth, hot flushes, stomach cramp are seen in the patient. Tell your doctor immediately ifyou see effects like chest pain, irregular heartbeats, swelling of breast,difficulty in urination because this can cause very serious damage to your body. Menstrual changes and sexual difficulties are also found I the females.
PRECAUTIONS: Tell your doctor if you have allergies towards the drug or have breast cancer. This medication taken in pregnancy if it clearly needed otherwise does not take the medication. Take your doctors advice before breast feeding the infants.
CDSCO APPROVAL: Cinnarizine + Domperidonetablets approved by CDSCO in India in 1992- February,
Domperidone SR tab approved by CDSCO in India in 16.01.2003,
Domperidone (10mg) + Activated dimethicone (125mg) chewable tablet approved by CDSCO in India in22.11.2005,
S(-) pantoprazole (as sodium) 20mg (E.C.) + Domperidone SR 30mg tablet approved by CDSCO in India in 30.08.2006,
Pantoprazole + Domperidone SR approved by CDSCO in India in 24.12.2004,
Rabeprazole Sodium 20mg + Domperidone 30mg S.R Capsule approved by CDSCO in India in
04.10.2004,
Dexrabeprazole 10mg (EC) + Domperidone 30mg SR Capsules approved by CDSCO in India in 09.06.2008,
Omeprazole(20mg) Enteric coated + Domperidone (30mg) (S.R.) capsule approved by CDSCO in India in 10.03.2005,
Domperidone tablets/suspension/oral drops/rectal suppositories approved by CDSCO in India in 1987-November,
Esomeprazole + Domperidone SR capsules approved by CDSCO in India in 2004
FORMULATIONS AVAILABLE IN MARKET:
Esomeprazole + Domperidone SR capsules
Domperidone tablets
Domperidone suspension
Domperidone oral drops
Pantoprazole + Domperidone SR tablets
Domperidone 10mg + Activated dimethicone 125mg chewable tablets
Omeprazole 20m) Enteric coated + Domperidone 30mg (S.R.) capsules
Domperidone rectal suppositories
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese