FENOFIBRATE
Product Details:
- Shelf Life 3 Years
- HS Code 29420090
- Molecular Formula C20H21ClO4
- Storage Keep away from moisture
- Molecular Weight 360.83 g/mol GSM (gm/2)
- Medicine Name FENOFIBRATE
- Chemical Name FENOFIBRATE
- Click to View more
FENOFIBRATE Price And Quantity
- 1700 INR/Kilograms
- 1700.00 - 2800.00 INR/Kilograms
- 5 Kilograms
FENOFIBRATE Product Specifications
- 360.83 g/mol GSM (gm/2)
- Keep away from moisture
- Pharmaceutical Intermediates
- Powder
- 99 %
- 29420090
- FENOFIBRATE
- FENOFIBRATE
- 49562-28-9
- 3 Years
- Medicine Grade
- White to Off-White Solid
- Fenofibrate comes in the anti lipidemic class of medication. Fenofibrate lowers the LDL (low density lipoproteins) which is also called bad cholesterol. Fenofibrate increase the natural substance which cause degradation of the fats in the blood.
- C20H21ClO4
FENOFIBRATE Trade Information
- INDIA
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Dadra and Nagar Haveli Chandigarh Himachal Pradesh Andaman and Nicobar Islands Uttarakhand Daman and Diu Lakshadweep Chhattisgarh South India Nagaland East India Andhra Pradesh Assam Arunachal Pradesh Bihar Goa Haryana Jammu and Kashmir Jharkhand Karnataka Madhya Pradesh Mizoram Meghalaya Manipur Punjab Pondicherry Rajasthan Sikkim Tamil Nadu Telangana Tripura West Bengal Maharashtra Delhi Gujarat Uttar Pradesh North India Kerala Central India Odisha West India All India
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceutical and our group companies Niksan healthcare are the one of largest manufacturer, exporter and supplier of Fenofibrate formulations and Fenofibrate API in Ankleshwar, Gujarat, India. We are supplying the best quality of Fenofibrate API all around the India as well as in the whole world. Our product Fenofibrate praised by our clients and also by the other companies.
Niksan Pharmaceutical is the world leading manufacturing and supplying company of the Fenofibrate products and API.
Thus we are Indian based company we supplying our best quality products of Fenofibrate to every states of India for many years like Jammu and Kashmir, Kerala, Punjab, Rajasthan, Andhra Pradesh ,Haryana, Telangana, Bihar, Karnataka, Delhi, Tamil Nadu, Odisha, Maharashtra,West Bengal, Uttar Pradesh, Gujarat, Madhya Pradesh, Himachal Pradesh, Goa,Chhattisgarh etc.
Niksan Pharmaceutical also export huge quantity of best quality Fenofibrate API products to the other nations like Puerto Rico,Philippines, United State, Nepal, Singapore, Lebanon, Indonesia, India,Bangladesh, Canada, United Arab Emirates, Sri Lanka, France, Jordan, Taiwan ,Malaysia, Vietnam, Thailand, Saudi Arabia, Australia , Pakistan, Iraq, SouthKorea, Hong Kong, Ireland, United Kingdom Algeria, Egypt, Belgium, Iran,Israel, Mexico, Germany, Turkey, Japan, Brazil and many south easterncountries.
Fenofibrate comes in the anti lipidemic class of medication. Fenofibrate lowers the LDL (low density lipoproteins) which is also called bad cholesterol. Fenofibrate increase the natural substance which cause degradation of the fats in the blood.
SYNONYMS: Fenofibrat, Fenofibrato, Fenofibratum,Finofibrate, Procetofen.
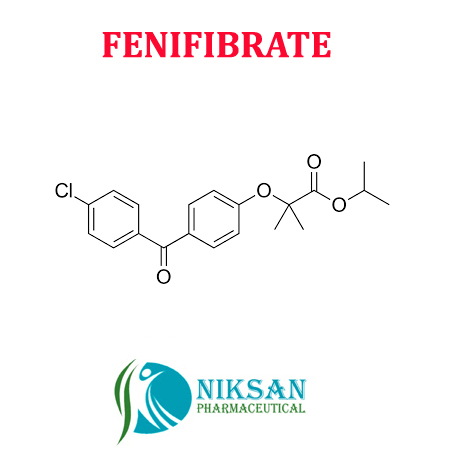
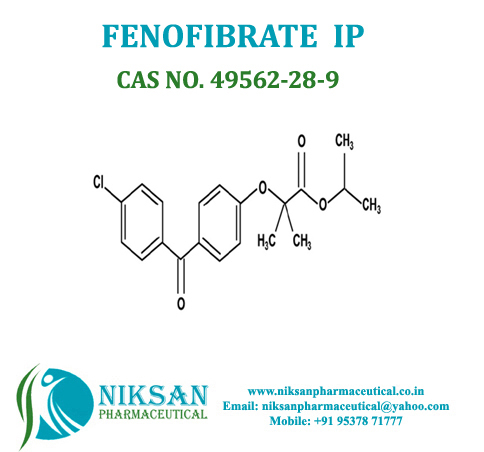
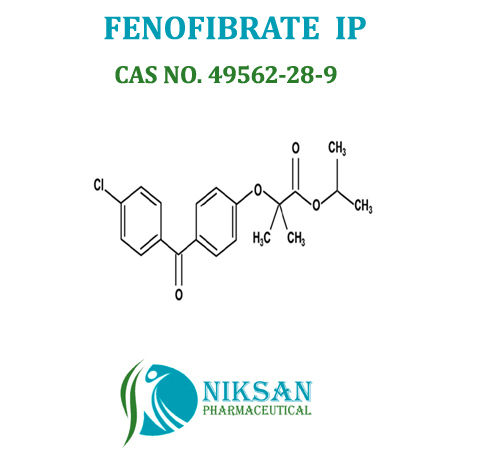
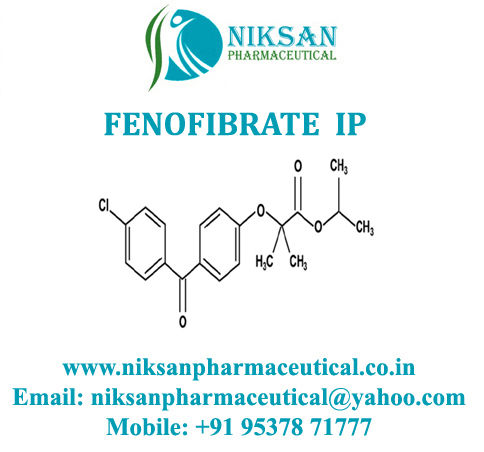
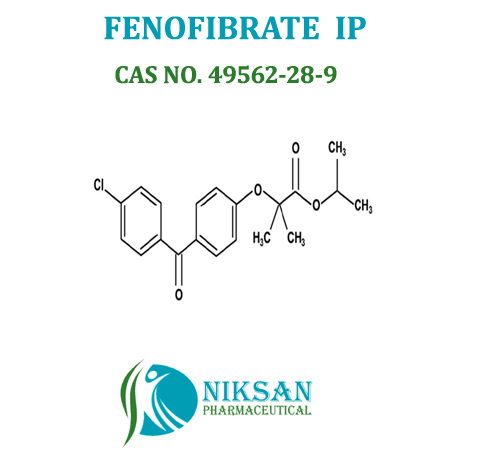
IUPAC NAME: Propan-2-yl2-[4-(4-chlorobenzoyl) phenoxy]-2-methylpropanoate
CAS NO: 49562-28-9
FORMULA: C20H21ClO4
MOLECULAR MASS: 360.83 g/mol
STORAGE CONDITIONS: Store in cool and dry place,keep away from direct heat and light. Do not store in bathroom or kitchen. Keep away from children and pet.
HOW TO USE: Take Fenofibrate by mouth onetime per day with or without food. Take medicine with food or milk if you feel stomach upset. Take your doctors advice if you have any confusion about medicine
HOW FENOFIBRATE WORKS: Fenofibrate lowers the bad cholesterol level by increasing the development of natural enzymes who breaks the fat or LDL in the blood. Fenofibrate lowers the bad cholesterol like LDL and triglyceride and increase the good cholesterol like HDL in body.
PHARMACOKINETICS: Fenofibrate rapidly absorbed in the body after the oral administration. It takes Fenofibrate 4-6 hours to reach the peak plasma concentration. Almost 99% of Fenofibrate binds with the blood plasma proteins. The half-life of Fenofibrate is about 19-27 hours in the normal patient and 143 hours in the patient with renal failure. The 60-88%of Fenofibrate eliminate through urination and 5-25% if Fenofibrate eliminate by the feces.
SIDE EFFECTS OF FENOFIBRATE: There are no many side effects of Fenofibrate is seen in the patient. Some side effects like kidney stone and liver problems are seen in some patients. Tell your doctor if you see some side effects like nausea and vomiting, stomach or intestinal pain, abdominal irritation, yellow eyes or skin and black urine. This drug rarely because effects like muscle pain, weakness in body and kidney problems.
PRECAUTIONS: The main precaution is keep check your HDL level. Tell your doctor if you have allergic reactions toward the Fenofibrate medication or its metabolite. If you have and disease like kidney problem,abdominal problem, liver problem or heart problem tell your doctor before writing the prescription. Do not take medication if you are in pregnancy period or in lactation period.
CDSCO APPROVAL: Rosuvastatin Calcium eq. toRosuvastatin 20mg/20mg + Fenofibrate BP 67.5mg/145mg Tablets approved by CDSCO in Indian in 24.08.2011,
Atorvastatin + Fenofibrate approved by CDSCO in Indian in 24.12.2004,
Atorvastatin 5mg + Fenofibrate 160mg (additional strength)approved by CDSCO in Indian in 01.12.2006,
Atorvastatin Calcium (10mg) + Fenofibrate (145mg) (addl. strength)approved by CDSCO in Indian in 30.10.2007,
Ezetimibe 10 mg + Fenofibrate 145 mg Film coated tablets approved by CDSCO in Indian in 01.09.2009,
FDC of Atorvastatin + fenofibrate approved by CDSCO in Indian in 01.08.2006,
Rosuvastatin 5/10/20mg + Fenofibrate 67/145/160mg approved by CDSCO in Indian in 23.08.2010
Choline Fenofibrate DR Capsule 45mg/135mg & DR Tablet 45mg/135mg. approved by CDSCO in Indian in 01.11.2012,
Rosuvastatin Calcium IP 5mg/10mg + Fenofibrate BP 160mg/160mg tablet approved by CDSCO in Indian in 29.12.2010,
Atorvastatin 10mg + Fenofibrate 80mg tablet (AdditionalIndication) 25.01.2008,
Metformin HCl ER 500mg + Fenofibrate 80mg/160mg tablet approved by CDSCO in Indian in 25.01.2008,
Fenofibrate capsules approved by CDSCO in Indian in 22.12.1999,
Ezetimibe 10mg + Fenofibrate 160mg tablet approved by CDSCO in Indian in 04.04.2007
FORMULATIONS AVAILABLE IN MARKET:
Fenofibrate 45mg tablets
Fenofibrate 35mg tablets
Fenofibrate 67mg tablets
Ezetimibe 10mg + Fenofibrate 160mg tablets
Fenofibrate capsules
Atorvastatin 10mg + Fenofibrate 80mg tablets
Rosuvastatin Calcium IP 5mg + Fenofibrate BP 160mg tablets
Rosuvastatin Calcium IP10mg + Fenofibrate BP 160mgtablets
Ezetimibe 10 mg + Fenofibrate 145 mg Film coated tablets
Atorvastatin 5mg + Fenofibrate 160mg tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese