GLIMEPIRIDE
Product Details:
- Shelf Life 3 Years
- HS Code 29420090
- Storage Room Temperature
- Molecular Formula C24H34N4O5S
- Molecular Weight 490.62 g/mol GSM (gm/2)
- Medicine Name GLIMEPIRIDE
- Chemical Name GLIMEPIRIDE
- Click to View more
GLIMEPIRIDE Price And Quantity
- 5 Kilograms
GLIMEPIRIDE Product Specifications
- GLIMEPIRIDE
- GLIMEPIRIDE
- 93479-97-1
- Medicine Grade
- Powder
- 490.62 g/mol GSM (gm/2)
- 3 Years
- 99 %
- White to Off-White Solid
- Other
- C24H34N4O5S
- 29420090
- Room Temperature
- Glimepiride come in the sulfonylurea class of drug which acts by increasing the body insulin which cause decrease in blood sugar.
GLIMEPIRIDE Trade Information
- SAHAR AIR CARGO
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Chandigarh Dadra and Nagar Haveli Tripura Manipur Andaman and Nicobar Islands Uttarakhand South India North India East India West India Andhra Pradesh Assam Arunachal Pradesh Bihar Chhattisgarh Daman and Diu Gujarat Goa Haryana Himachal Pradesh Jammu and Kashmir Jharkhand Karnataka Kerala Lakshadweep Maharashtra Mizoram Meghalaya Nagaland Punjab Pondicherry Rajasthan Sikkim Tamil Nadu Telangana Uttar Pradesh West Bengal Delhi Madhya Pradesh Central India Odisha All India
- FDCA, GMP, GLP AND ISO
Product Description
Niksan Pharmaceutical and our group companies Niksan healthcare are the one of largest manufacturer and supplier of Glimepiride formulations and Glimepiride API in Ankleshwar, Gujarat, India. We are supplying the best quality of Glimepiride API all around the India as well as in the whole world. Our product Glimepiride praised by our clients and also by the other companies.
Niksan Pharmaceutical is the worlds leading manufacturing and supplying company of the Glimepiride products and API.Thus we are Indian based company we supplying our best quality products to every states of India for many years like Jammu and Kashmir, Kerala, Punjab,Rajasthan, Andhra Pradesh, Haryana, Telangana, Bihar, Karnataka,Delhi, TamilNadu, Odisha, Maharashtra, West Bengal, Uttar Pradesh, Gujarat, Madhya Pradesh,Himachal Pradesh, Goa, Chhattisgarh etc.
Niksan Pharmaceutical also exports huge quantity of best quality Glimepiride API products to the other nations like Puerto Rico, Indonesia, Nepal, Ghana, United States, Philippines, Jordan, Pakistan, Nigeria, Sri Lanka, United Arab Emirates, South Africa, Bangladesh, Netherlands, Taiwan, Iraq, Saudi Arabia, Venezuela, Hong Kong, Singapore, Vietnam, Egypt, South Korea, Algeria,Morocco, France, Israel, Australia, United Kingdom, Canada, Malaysia, Italy,Thailand, Colombia, Mexico, Argentina, Spain, Germany, Brazil,Japan and many south eastern countries.
Glimepiride is the type of drug which is used in the treatment of the type II diabetes mellitus .Glimepiride is basically the oral dosage form. With the diet and proper exercise and treatment of Glimepiride works more effectively. Glimepiride patented in year 1979 and then it is approved for the medical use in the year 1995.
Glimepiride come in the sulfonylurea class of drug which acts by increasing the body insulin whichcause decrease in blood sugar.
SYNONYMS OF GLIMEPIRIDE:Glimepirida, , Glimepiride, Glimepiridum.
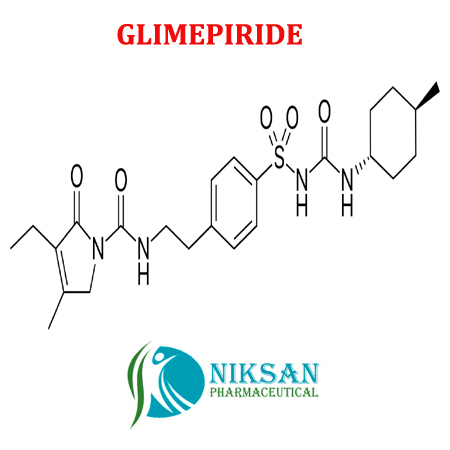
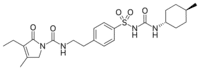
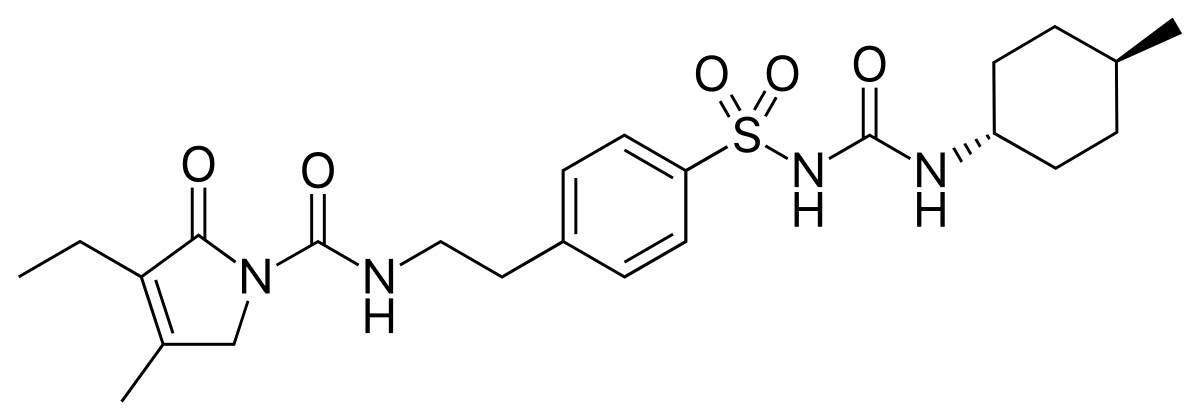
IUPAC NAME: 3-Ethyl-4-methyl-N-[2-(4-{[(trans-4-methylcyclohexyl)carbonyl] sulfonyl} phenyl) ethyl]-2-oxo-2, 5-dihydro-1H-pyrrole-1-Carboxamide
CAS NO:93479-97-1
FORMULA: C24H34N4O5S
MOLECULAR MASS:490.62g/mol
HOW TO USE: Read the leaflet of the Glimepiride. the medicine is only taken one time a day so take the medicine in morning orally with breakfast.to take the most benefit of Glimepiride take it regularly at a same time for days. If you are already taking other diabetic medicines take your doctors advice to how to use the Glimepiride medication.
HOW GLIMEPIRIDE WORKS: Glimepiride main work is to reduce the blood sugar level by stimulating the insulin from pancreatic beta cells. By this process the peripheral glucose uptake and by this the blood sugar level decreased by the Glimepiride.
CONTRAINDICATIONS OF GLIMEPIRIDE :If you have any allergy, tell your doctor before taking these medicines and other inactive ingredients which can cause allergic reactions. Because of the action of the drug the blood sugar level will decrease by this the blurred vision and drowsiness effects are seen so do not take this medicine while driving or doing any machinery works which can cause hazardous reaction. Do not go out in sun because this medicine can make you sensitive to the sun.
PHARMACOKINETICS OF GLIMEPIRIDE: Glimepiride is taken orally and will easily absorb after the administration in 1 hour and reach the blood peak plasma concentration within 3 hours. The plasma protein binding of Glimepirideis more than 99.5%. The drug will metabolise completely in liver. Basically the half-life of Glimepiride is 5-8 hours but it can increase by taking multiple dosages.The excretion of Glimepiride is mainly (60%) done by urination and the other is done in feces.
SIDE EFFECTS OF GLIMEPIRIDE: The normal side effects of Glimepiride are nausea and upset stomach. Contact your doctor if any side effect like weakness, yellowing of eye/skin, dark urine, bleeding, infections, mood swings occurs. The medicine cause loss of blood sugar so weakness in body,shaking of arms and feet, hunger, blurred vision, unconsciousness type of effects are seen in the patient. If you notice symptoms like rash, allergy, swelling of face, itching, and trouble in breathing contact your doctor immediately.
PRECAUTIONS OF GLIMEPIRIDE: The medicine cause loss of blood sugar so kindly avoids the heavy exercise. Do not drink alcohol it can cause low blood sugar level. Because Glimepiride reduce the blood sugar level kindly avoids the activity like driving or exercising or operating heavy machineries. Discuss the medication plan with your doctor before taking the medication, and always take medicine in time.
CDSCO APPROVAL: Glimepiride 1mg/2mg/3mg/4mg tablets are approved by CDSCO in India in 24.01.1999.
Pioglitazone HCL + glimepiride tablet is approved by CDSCO in India in 25.10.2002.
Glimepiride + metformin SR tablet is approved by CDSCO in India in 13.11.2002.
Rosiglitazone + glimepiride tablet is approved by CDSCO in India in 14.11.2002.
Glimepiride + rosiglitazone tablet is approved by CDSCO in 12.12.2003.
FDC of Glimepiride (1mg/2mg) + Pioglitazone (15mg) + Metformin(500mg E.R) Tablet is approved by CDSCO in India in 12.08.2005.
Glimepiride 1mg/2mg. + Metformin SR 1000mg tablet (additionalstrength) is approved by CDSCO in 08.06.2007.
FORMULATIONS AVAILABLE IN MARKET:
Glimepiride 1mgtablets
Glimepiride 2mgtablets
Glimepiride 3mgtablets
Glimepiride 4mg tablets
Pioglitazone HCL + glimepiride tablets (15mg+1/2mg)
Glimepiride + metformin (1/2mg+500mg)
Rosiglitazone + glimepiride (2mg+1mg)
Glimepiride + rosiglitazone (1/2mg+30mg)
Glimepiride (1mg/2mg) + Pioglitazone (15mg) + Metformin (500mgE.R) Tablet
Glimepiride 1mg/2mg. + Metformin SR 1000mg tablet (additional strength)
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese