- Home Page
- Company Profile
-
Our Products
- Active Pharmaceuticals Ingredients (API)
- ESOMEPRAZOLE
- CILNIDIPINE
- BENIDIPINE
- TORSEMIDE
- IVERMECTIN
- ADAPALENE
- SOLIFENACIN SUCCINATE
- ARTEMETHER
- NORETHINDRONE
- FENBENDAZOLE
- BRIVARACETAM
- Quetiapine
- BECLOMETHASONE DIPROPIONATE
- EDARAVONE
- PRIMIDONE
- MELATONIN
- BENFOTIAMINE
- METOCLOPRAMIDE
- CLOSANTEL SODIUM
- LERCANIDIPINE
- SILODOSIN
- SERTRALINE
- NORETHISTERONE ACETATE
- ALLOPURINOL
- FEBUXOSTAT
- OLMESARTAN
- FINASTERIDE
- FENOFIBRATE
- PREGABALIN
- ORNIDAZOLE
- ETORICOXIB
- TRIAMCINOLONE
- ROSUVASTATIN
- BISOPROLOL
- VOGLIBOSE
- TRAZODONE
- TENELIGLIPTIN
- ATORVASTATIN
- LABETALOL
- CELECOXIB
- CLARITHROMYCIN
- FLUOCINOLONE ACETONIDE
- NICORANDIL
- NATEGLINIDE
- MICONAZOLE NITRATE
- TERBINAFINE
- DIACEREIN
- AMISULPRIDE
- PRAZIQUANTEL
- PROGESTERONE
- LEVOSULPIRIDE
- LULICONAZOLE
- NEBIVOLOL
- PREDNISOLONE
- CETIRIZINE
- FLUOROMETHOLONE
- AZILSARTAN
- TIZANIDINE
- NIFEDIPINE
- ERYTHROMYCIN
- FEBANTEL
- ITOPRIDE
- MOXIFLOXACIN HCL
- CLOBETASOL PROPIONATE
- AMBROXOL
- DROTAVERINE
- FEXOFENADINE
- DOMPERIDONE
- FLUCONAZOLE
- LEVETIRACETAM
- LUMEFANTRINE
- Torsemide
- DONEPEZIL
- DOXOFYLLINE
- GABAPENTINE
- GLIMEPIRIDE
- ONDANSETRON
- PIROXICAM
- LOPERAMIDE
- METHYLPREDNISOLONE
- RASAGILINE
- BILASTINE
- CILOSTAZOL
- TAMSULOSIN
- CITICOLINE
- RIVAROXABAN
- LITHIUM CARBONATE
- MEMANTINE
- LEFLUNOMIDE
- DABIGATRAN
- PANTOPRAZOLE
- LACOSAMIDE
- QUETIAPINE
- SITAGLIPTIN
- Norethindrone Acetate
- Cilostazol
- Dapoxetine
- Minoxidil
- Nandrolone Phenylpropionate
- Orlistat
- Sertraline
- Silodosin
- Tadalafil
- DEFLAZACORT
- DESLORATADINE
- LORNOXICAM
- METOPROLOL
- Pharma Intermediates
- 5-Chloro-2-Nitro Aniline
- 1-(3-Methyl-1-phenyl-5-pyrazolyl)piperazine acetate salt
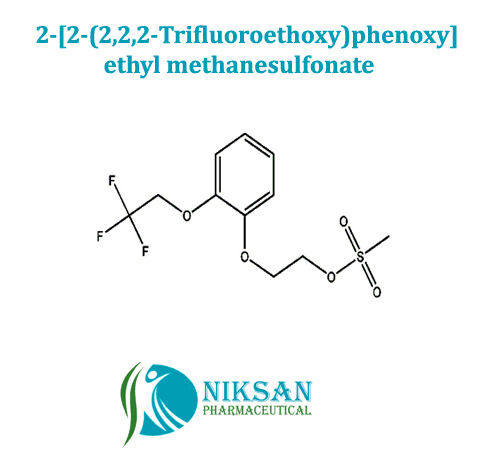
- 2-[2-(2,2,2-Trifluoroethoxy)phenoxy]ethyl methanesulfonate
- 4-Chloro-3-Pyridine Sulfonamide
- 5-((2R)-2-AMINOPROPYL)-2-METHOXYBENZENE SULFONAMIDE
- 2-Methoxyethyl-(3-Nitrobenzyledene) Acetoacetate
- IMIDAZOL-1-YL-ACETONITRILE
- (R)-(-)-3-Carbamoymethyl-5-methylhexanoic acid
- BARNIDIPINE INTERMEDIATES
- CILOSTAZOL INTERMEDIATES
- (2R)-rel-6-Fluoro-3,4-dihydro-2-(2R)-2-oxiranyl-2H-1-benzopyran
- (2R)-rel-6-Fluoro-3,4-dihydro-2-(2S)-2-oxiranyl-2H-1-benzopyran
- 5-2r-2-aminopropyl-1-3-benzoyloxy-propyl-2-3-dihydro-1h-indole-7-carbonitrile-2r-3r-2-3-dihydroxybutanedioate
- TORSEMIDE INTERMEDIATES
- (2S)-4-Oxo-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester
- 1-(3-Methyl-1-phenyl-5-pyrazolyl) piperazine
- 2, 6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic Acid
- BENIDIPINE INTERMEDIATE
- LULICONAZOL INTERMEDIATES
- PREGABALIN INTERMEDIATE
- TAMSULOSIN INTERMEDIATES
- 4-(3'-Methylphenyl)amino-3-pyridinesulfonamide
- Febantel Intermediates
- Cilnidipine Intermediates
- 3-Carbamoymethyl-5-methylhexanoic acid
- 2(2- ETHOXY PHENOXY ) ETHYL BROMIDE
- SILODOSIN INTERMEDIATE
- 2-methoxy-N-[2-nitro-5-(phenylthio)phenyl]Acetamid
- 3-amino Crotonicacid Cinnamyl Ester
- TENELIGLIPTIN INTERMEDIATES
- NEBIVOLOL INTERMEDIATES
- SILODOSIN INTERMEDIATES
- Nicardipine Intermediate
- Dapoxetine IP
- MEPIRODIPINE INTERMEDIATE
- MANIDIPINE INTERMEDIATE
- LERCANIDIPINE INTERMEDIATE
- 5-(4-Chlorobutyl)-1-cyclohexanyl tetrazole
- 1-Benzyl-3-hydroxypiperidine
- FENBENDAZOLE INTERMEDIATES
- 1,3-Bis(Methoxycarbonyl)-2-Methyl-2-Thiopseudouera
- 6-Hydroxy-2(1H)-3,4-dihydroquinolinone
- (S)-2-Chloro-1-(2,4-dichlorophenyl)ethanol
- 2-Nitro 5-Phenyl Mercapto Aniline
- 3-Nitrobenzaldehyde
- Pranidipine Intermediate
- Pharmaceutical Formulation
- Chlorthalidone 12.5 Mg Tablets
- Rabeprazole Sodium (Ec) Levosulpride (Sr) Capsules
- Haemitinic Capsules
- SUNSCREEN CREAM 50 SPF
- Levosulbutamol Ambroxol & Guaphensin Syrup
- Ibuprofen 200 Mg Tablets
- Ibuprofen 400 Mg Tablets
- Cyproheptadine 1.5 Mg Tablets
- Itraconazole Dusting Powder
- Doxycycline Hcl 100 Mg Tablets
- Mupirocin 2.0 % W/W Ointment
- Stanozolol Usp Powder
- Glipezide 2.5 Mg Tablets
- Naproxen Sodium 275mg Tablets
- Labetalol 100 Mg Tablets
- Calcium Carbonate Magnesium Zinc Vitamin D3 Suspension
- Paracetamol 500 Mg Caffeine 65 Mg Tablets
- Nimesulide 100 Mg Paracetamol 500 Mg Tablets
- Montelukast Levocetrizine Syrup
- Carbonyl Iron Folic Acid Cyanocobalamine Zinc Syrup
- Artemether Capsules 40 Mg
- Amoxycillin 250 Mg Capsules
- Amoxycillin Trihydrate Dicloxacillin Capsules
- Fluconazole & Chlorhexadine Hcl Powder
- Fluconazole 0.5 % Cream
- Fluconazole 50 Mg Capsules
- Megaldreate Simethicone Domperidone Suspension
- Amoxycillin Potasium Clavunate Syrup
- Ofloxacin Metronidazole Simethicone Suspension
- Co-trimoxazole 120 Mg Tablets
- Etoricoxib 90 Mg Tablets
- Norfloxacin 400 Mg Tinidazole 600 Mg Tablets
- Paracetamol 100 Mg Dispersible Tablets
- Bisacodyl 5 Mg Tablets
- Paracetamol 500 Mg Domperidone 20 Mg Tablets
- Paracetamol 500 Mg Tablets
- Ketoconazole 200 Mg Tablets
- Nebivolol 5 Mg Tablets
- Diltiazem 90Mg Sustain Release Tablets
- Calcium Carbonate Vitamin D3 125Iu Suspension
- Silodosin 8 Mg Capsules
- Enalapril Maleate 10 Mg Hydorchlorthiazide 15 Mg Tablets
- Diltiazem 30Mg Tablets
- Diltiazem 240 Mg Sustain Release Tablets
- Esomeprazole Clarithromycin Amoxycillin Tablets
- Metoprolol Tartrate 50 Mg Sustain Release Tablets
- Losartan Potassium 50 Mg Amlodipine 5 Mg Tablets
- Losartan Potassium Hydrochlorothiazide Tablets
- Duloxetine Hcl 20Mg Tablets
- Metformin Hcl 500 Mg Tablets
- Diltiazem 60Mg Tablets
- Metformin Hcl 1000 Mg Tablets
- Tetracycline 500 Mg Tablets
- Voglibose 0.3 mg Metformin 500 mg tablets
- Cyproheptadine Hydrochloride Syrup
- Gabapentin 600 Mg Tablets
- Glimepride 2 Mg Tablets
- Glimepride 1 Mg Tablets
- Cetirizine 5 Mg Tablets
- Chlorthalidone 100 Mg Tablets
- Dexamethasone 1 Mg Tablets
- Dihydralazine 25Mg Tablets
- Glipezide 5 Mg Tablets
- Glibenclamide 5 Mg Metformin 500 Mg Tablets
- Diclofenac 50Mg Paracetamol 500Mg Tablets
- Drotaverine 80Mg Tablets
- Amisulpride 50 mg tablets
- FEBUXOSTATE Tablets 40 Mg
- FEBUXOSTATE Tablets 80mg
- Levosulpride 50 mg tablets
- FEBENTEL 150 Mg tablets
- Lansoprazole 15 Mg Tablets
- Lansoprazole 30 Mg Domperidone 10 Mg Tablets
- Fusidic Acid Beclomethsone Dipropionate Cream
- Fluconazole 150 Mg Tinidazole 1000 Mg Tablets
- Piracetam Tablets 400 Mg
- Finasteride 5mg Tablets
- Ketoconazole Zinc Pyrithione Zpto Shampoo
- Ketoconazole 2 % V/V Shampoo
- Norfloxacin 400 Mg Metronidazole 500 Mg Tablets
- Flavoxate Hcl 200Mg Tablets
- Ofloxacin 100 Mg Tablets
- Flunarizine Domperidone Paracetamol Tablets
- Ofloxacin 200 Mg Tinidazole 600 Mg Tablets
- Bacitracin Zinc Neomycin Polymixin B Sulphate Ointment
- Diclofenac Sodium 75 Mg Tablets
- Rabeprazole Sodium 20 Mg Tablets
- Sertraline Hydrochloride 25 Mg Tablets
- Dicyclomine 10 Mg Diclofenac Potassium 50 Mg Tablets
- Griseofulvin Tablets 125 Mg
- Domperidone 10 Mg Tablets
- Isosorbide Dinitrate 20 Mg Tablets
- Isosorbide Dinitrate 40 Mg Tablets
- Isosorbide Mononitrate 5 Mg Tablets
- Isosorbide Mononitrate 10 Mg Tablets
- Nebivolol 2.5 Mg Tablets
- Lisinopril 5 Mg Amlodipine 5 Mg Tablets
- Metoclopramide Hcl 10 Mg Tablets
- Rosuvastatin 5mg Tablets
- Metoprolol Tartrate 25Mg Sustain Release Tablets
- Silodosin 4Mg Capsules
- Frusemide 40 Mg Amiloride 5 Mg Tablets
- Carvedilol 3.125 Mg Tablets
- Carvedilol 12.5 Mg Tablets
- Clopidogrel Bisulphate 75 Mg Tablets
- Metoprolol Tartrate 100 Mg Sustain Release Tablets
- Olmesaratan 20mg Tablets
- Telmisartan 20 Mg Tablets
- Telmisartan 40 Mg Tablets
- Telmisartan 40 Mg Hydrochlorothiazide 12.5 Mg Tablets
- Torsemide 10mg Tablets
- Carvedilol 6.25 Mg Tablets
- Hydrochlorothiazide 25Mg Tablets
- Bosentan 125 mg Tablets
- Frusemide 40 Mg Spirionlactone 50 Mg Tablets
- Ciprofloxacin 100 Mg Tablets
- Diclofenac Sodium 50 Mg Tablets
- Loperamide Hcl 2 Mg Tablets
- Flunarizine 5 Mg Tablets
- Praziquantel Pyrantel Pamoate And Fenbendazole Bolus
- Praziquantel Pyrantel Pamoate And Febentel Tablet
- Voglibose 0.3 mg tablets
- Azilsartan 40mg Tablets
- Doxylamine Succinate 10Mg Pyridoxine Hcl 10 Mg Tablets
- CILOSTAZOLE 100 MG Tablets
- Ofloxacin Ornidazole 30 Ml Suspension
- Cefixime Ofloxacin Dry Syrup
- Febuxostate Eye Drop
- Ciprofloxacin 500Mg Ornidazole 500Mg Tablets
- Bromhexine Guaiphenesin Terbutaline Sulphate 100 Ml Syrup
- Ornidazole 500mg Tablets
- Diloxanide 500Mg Tablets
- Sparfloxacin 100 Mg Tablets
- Nicorandil 5 Mg Tablets
- Nicorandil 10 Mg Tablets
- Levofloxacin Ornidazole Dry Syrup
- Povidone Iodine Ornidazole Ointment
- Dicyclomine 10 Mg Mefenamic Acid 250 Mg Tablets
- Levofloxacin 500 Mg Ornidazole 1Gm Tablets
- Pregabalin Capsules 50 Mg
- Levofloxacin 250 Mg Tablets
- Levofloxacin 500 Mg Tablets
- Mefenamic Acid Paracetacetamol Suspension
- Mefenamic Acid 500 Mg Tablets
- Moxifloxacin Hcl 400 Mg Tablets
- Mefloquine 250 Mg Tablets
- Metformin Hcl 500 Mg Sustain Release Tablets
- Vitamin- C 50mg Tablets
- Pepsin Diastase Tablets
- Hydrochlorothiazide 12.5Mg Tablets
- Isosorbide Dinitrate 10 Mg Tablets
- Fluocinolone Miconazole Neomycin Cream
- Repaglinide 2 Mg Tablets
- Glipezide 5 Mg Metformin 500 Mg Tablets
- Erythromycin Estolate 250 Mg Tablets
- Pregabalin Capsules 75 Mg
- Salbutamol 2 Mg Tablets
- Clotrimazole Vaginal 100 Mg Tablets
- Clotrimazole 1% W/W Dusting Powder
- Itraconazole 100 Mg Capsules
- Clobetasole Miconazole Gentamycin Zinc Oxide Lotion
- Haemitinic Syrup
- Diacerein Linseed Oil Methylsalicylate Menthol Ointment
- Pantoprazole 40mg Levosulpiride 75mg (Sr) Capsules
- Ferric Ammonium Citrate Vitamin B-12 Folic Acid Syrup
- Theophylline 400 Mg Controlled Release Tablets
- Theophylline 200 Mg Salbutamol 4 Mg Tablets
- Theophylline 200 Mg Tablets
- Cefpodoxime Dry Syrup
- Febendazole 3GM BOLUS
- Metronidazole 400mg Furazolidone Simethicone Tablets
- Etoricoxib Thiocolchicoside tablets
- Ferrous Fumarate Folic Acid Vit B12 Zinc Sulphate Tablets
- Choline Salicylate Lignocaine Hcl Benzalkonium Gel
- SUCRALFATE LIGNOCAINE CREAM
- Gabapentin Lignocaine Methyl & Propyl Paraben Ointment
- Cefpodoxime Potasium Clavunate Dry Syrup
- Metronidazole 200 Mg Tablets
- Clotrimazole Beclomethasone Neomycin Cream
- Clindamycin Adapalene Gel
- Clindamycin Phosphate Gel
- Clindamycin Nicotinamide Gel
- Glucosamine Diacerein Capsicum Oleoresin Menthol Gel
- Glycerin 15 % W/w Cream
- Diclofenac Linseed Oil Thiocolchicoside Menthol Gel
- Piroxicam Gel Bp
- Loperamide Hcl 2 Mg Capsule
- Beclomethasone Propionate Miconazole Neomycin Cream
- Clobetasol Propionate Clotrimazole Neomycin Cream
- Simvastatin 10 Mg Tablets
- Simvastatin 5 Mg Tablets
- Cinnarizine 75 Mg Tablets
- Cinnarizine 25 Mg Tablets
- Aceclofenac Paracetamol Suspension
- Lumefantrine 120Mg Artemether 20Mg Tablets
- Diclofenac Linseed Oil Benzyl Alcohol Gel
- Diclofenac Linceed Oil Methyl Salicylate Menthol Gel
- Benzoyl Peroxide 2.5 % W/w Gel
- Adapalene Gel
- Tretinoin Cream
- Ciprofloxacin Cream
- Permethrin Soap
- Alpha Amylase Papain 30 Ml Syrup
- Cholecalciferol Granules 60,000 I.U
- MOMETASONE FUROATE CREAM
- Cefixime Dry Syrup
- Paracetamol Phenylepherine Cetrizine Zinc Gluconate Syrup
- Betamethasone and Gentamycin Cream
- Vitamin E Acetate Cream
- Vitamin D3 Drops
- Itraconazole 200 Mg Capsules
- Bisoprolol 2.5 Mg Tablets
- Bisoprolol 2.5 Mg Amlodipine 5 Mg Tablets
- Aripiprazole Tablet 5 Mg
- Atorvastatin 20 Mg Tablets
- Atorvastatin 10 Mg Tablets
- Roxithromycin 50 Mg Tablets
- Amlodipine 5Mg Atenolol 25Mg Tablets
- Atenolol 25 Mg Tablets
- Atenolol 50 Mg Tablets
- Atenolol Lostartan Tablets
- Atenolol 50Mg Nifedipine 20Mg Tablets
- Beta Carotene Vitamin A Vitamin E Vitamin C Tablets
- Amlodipine 2.5Mg Tablets
- Amlodipine Atorvastatin Tablets
- B Complex Multivitamin With L Lysine Syrup
- Betamethasone 0.5Mg Tablets
- Acetazolamide 250 Mg Tablets
- Salbutamol 4 Mg Tablets
- Tetracycline 250 Mg Capsules
- Ambroxol Salbutamol Syrup
- Praziquantel Pyrantel Pamoate And Fenbendazole Tablet
- Aceclofenac 200 Mg Tablets
- Aceclofenac 100 Mg Serratiopeptidase 15 Mg Tablets
- Calcium 250 Mg Vitamin D3 125 Iu Tablets
- Azithromycin 100 Mg Tablets
- Amlodipine 5 Mg Losartan 50 Mg Tablets
- Amlodipine 5 Mg Lisinopril 5 Mg Tablets
- Acarbose 100 Mg Tablets
- Cilnidipine 10 Mg Tablets
- Aceclofenac Tablets 100 Mg
- Aceclofenac 100 Mg Paracetamol 500 Mg Tablets
- Allopurinol 100 Mg Tablets
- Amiodarone 100 Mg Tablets
- Benidipine Hydrochloride 8 Mg Tablets
- Montelukast Sodium 5 Mg Tablets
- Repaglinide 1 Mg Tablets
- Prednisolone 5 Mg Tablets
- Aceclofenac Paracetamol Rabeprazole Tablets
- Drotaverine 40Mg Tablets
- Amlodipine 5 Mg Tablets
- Enalapril Maleate 5Mg Tablets
- Amiodarone 200 Mg Tablets
- Albendazole Chewable 200 Mg Tablets
- Amodiaquine 200 Mg Tablets
- Acyclovir 200 Mg Tablets
- Acarbose 50 Mg Tablets
- SERTACONAZOLE 2 % W/W CREAM
- Ondansetron 4 Mg Paracetamol 500 Mg Tablets
- Azathioprine 50 Mg Tablets
- Chlorhexidine Gluconate Salicylic Acid Soap
- Artemether 180 Mg Lumefantrine 1080 Mg Tablets
- Artesunate 100Mg Tablets
- Artesunate Sulphadoxin Pyrimethamine Tablets
- Artesunate 100 Mg Amodiaquine 270 Mg Tablets
- Artesunate 100 Mg Mefloquine 220 Mg Tablets
- Artesunate 50Mg Tablets
- Candesartan Cilexetil 16 Mg Tablets
- Candesartan Cilexetil 8 Mg Tablets
- Acetazolamide 250Mg Sustain Release Tablets
- Azithromycin Suspension
- Enalapril Maleate 10 Mg Tablets
- Norfloxacin 100 Mg Tablets
- Paracetamol Phenylepherine Cpm Sodium Citrate Syrup
- OFLOXACIN ORNIDAZOLE TERBINAFINE CLOBETASOL CREAM
- CLOTRIMAZOLE BECLOMETHOSONE NEOMYCIN CHLOROCRESOL CREAM
- Clobetasol Propionate Salicylic Acid Ointment
- Diclofenac Sodium 1 % W/w Gel
- Beclomethasone Propionate Clotrimazole Neomycin Cream
- Cefixime Potasium Clavunate Dry Syrup
- OFLOXACIN CLOBETASOL ORNIDAZOLE ITRACONAZOLE CREAM
- Ketoconazole 2 % W/w Cream
- Vitamin B1 B2 B6 B12 C Biotin Calcium Capsules
- Cinnarizine 15Mg + Domperidone 15 Mg Tablets
- Hydroquinone Tretinoin Momentasone Cream
- Montelukast Sodium 10 Mg Tablets
- Praziquantel Pyrantel Pamoate And Fenbendazole SUSPENSION
- Tetracycline 50 Mg Dispersible Tablets
- Amoxycillin Dry Syrup
- Azithromycin 200Mg Dry Syrup
- Ethamsylate 250 Mg Tablets
- Clarithromycin 125Mg Dry Syrup
- Famotidine 20 Mg Tablets
- Famotidine 40 Mg Tablets
- TELMISARTAN 40 MG RAMIPRIL 2.5 MG TABLETS
- Gabapentin 800 Mg Tablets
- SERTACONAZOLE NITRATE BECLOMETHASONE DIPROPIONATE CREAM
- Enzymes Syrup
- Fexofinadine Syrup
- Levocetrizine Phenylepherine Syrup
- Ibuprofen Paracetamol Tablets
- TACROLIMUS OINTMENT
- Glibenclamide 5 Mg Tablets
- Calamine With Vitamin E, Aloe Vera & Olive Oil Lotion
- Ketoconazole Soap 2% W/W
- Benzoyl Peroxide Clindamycin Phosphate Soap
- SODIUM FUSIDATE CREAM
- TERBINAFINE HYDROCHLORIDE CREAM
- Benzoyl Peroxide Cream
- Benzydamine Hcl Mouth Wash
- Lactic Acid Bacillus 60 Million Spores Capsule
- Losartan Potassium 50 Mg Tablets
- Lactobacilli Thiamine Riboflavin Pyridoxine Niacin Tablets
- Linezolid Dry Syrup
- Hydrocortisone Acetate Fusidic Acid Cream
- LULICONAZOLE 1 % W/W CREAM
- Clobetasol Propionate Miconazole Neomycin Zinc Cream
- Clotrimazole Ip 1% Soap
- Nimodipine 30 Mg Tablets
- FEBENTEL 75mg Tablets
- Eberconazole 1 % W/w Cream
- Doxycycline Dispersible 100Mg Tablets
- Venlafaxine 25mg Tablets
- Etoricoxib 60 Mg Tablets
- Lansoprazole 30 Mg Tablets
- Metoprolol Tartrate 25 Mg Amlodipine 5Mg Tablets
- Pyrazinamide Tablets 400 Mg
- Torsemide 20 Mg Tablets
- Frusemide 40 Mg Tablets
- Oral Rehydration Salts
- Losartan Potassium 25 Mg Tablets
- Folic Acid 1 Mg Tablets
- Multivitamin Multimineral 200 Ml Syrup
- Sucralfate Tinidazole Povidone Iodine Ointment
- Sucralfate Oxcetacain 60Ml Suspension
- Dextromethorphan Cpm Guaiphenesin & Ammonium Syrup
- Chloroquine 500Mg Tablets
- Chloroquine 250Mg Tablets
- Pantoprazole 20 Mg Tablets
- Pantoprazole 40 Mg Tablets
- Pantoprazole 30 Mg Ondonsetron 4 Mg Tablets
- SILVERSULPHADIAZINE AND CHLORHEXIDINE CREAM
- Lactic Acid Vaginal Wash
- Lycopene Multi Vitamins Multi Minerals 200 Ml Syrup
- Salicylic Acid Face Wash
- Dexrabeprazole Domperidone (Sr Pellets) Capsules
- Esomprazole 40mg Domperidone 20mg (Sr Pellets) Capsules
- Minoxidil Solution
- Mecobalamin 1500 Mcg Capsule
- Carbonyl Iron Folic Acid Zinc Sulphate Vitamin B12 Tablets
- Carbonyl Iron Folic Acid Zinc Vitaminb12 Vitamin C Capsules
- Folic Acid Zinc Sulphate Ferrous Sulphate Sr Capsules
- Biotin 5mg Folic Acid 5mg Capsule
- Iron Folic Acid Vitamin B12 Capsules
- Tinidazole 300 Mg Tablets
- Vitamin E With Aloe Vera Moisturising Soap
- Clarithromycin 250 Mg Tablets
- Hydrocortisone 100 Mg Tablets
- Dha-200mg Folic Acid 1.5mg Capsule
- Rosuvastatin 10 Mg Tablets
- Meloxicam 15 Mg Tablets
- Clindamycin Benzoyl Peroxide Gel
- Gabapentin 300 Mg Mecobalamin 500 Mcg Tablets
- Aceclofenac Linseed Oil Methyl Salicylate Menthol Gel
- cilecoxib 100 mg Capsules
- Montelukast Sodium 10 Mg Bambuterol 10 Mg Tablets
- Diclofenac Aloe Vera Methyl Salicylate Menthol Gel
- Piroxicam 20 Mg Tablets
- Febendazole - 150 MG TABLETS
- Praziquantel Pyrantel Pamoate And Fenbendazole SYRUP
- Cyproheptadine Tricholine Citrate Sorbitol Syrup
- CLOBETASOL PROPIONATE CREAM
- Clotrimazole Vaginal Cream
- Paracetamol Cetrizine Hcl Phenylephrine Hcl Suspension
- Fusidic Acid Cream
- Azithromycin 250 Mg Capsules
- Diclofenac Thiocolchicoside Capsules
- Omeprazole 20 Mg Domperidone 10 Mg Capsules
- Pantoprazole Domperidone Prologed Releases Capsules
- Dicyclomine Hydrochloride Pediatric Drops
- Paediatric Haemitinic Drops
- Ambroxol Guaiphenesin Levosalbutamol Oral Drops
- Enzymes Drops
- Phenylephrine Chlorpheniramine Maleate Paracetamol Drops
- Ketoconazole Idhq Tolnaftate Gentamicin Clobetasol Cream
- Paracetamol Suspension Bp
- Ambroxol Levocetrizine Syrup
- MICONAZOLE CREAM
- Artemether Lumifentrin Dry Syrup
- Cefixime Azithromycin Dry Syrup
- POVIDONE IODINE USP OINTMENT
- Levocetrizine Syrup
- Salbutamol Sulphate Cholin Theophylline Menthol Syrup
- Amorolfine 0.25 % W/w Cream
- Ambroxol Guaiphenesin Terbutaline Sulphate Syrup
- Dextromethorphan Chlorpheniramine Phenyephrine Syrup
- Omeprazole 20 Mg Capsules
- Nimesulide 100 Mg Tablets
- Ondansetrone 4 Mg Tablets
- Ondansetrone 8 Mg Tablets
- Dicyclomine 20 Mg Paracetamol 500 Mg Tablets
- Ofloxacin 400 Mg Ornidazole 600 Mg Tablets
- Salicylic Acid Ip Sulphure Usp Soap
- Mefenamic Acid 500 Mg Paracetamol 450 Mg Tablets
- Terazosin 2 Mg Tablets
- Pregabalin 75 Mg Methylcobalamin 750 Mcg Tablets
- Doxofylline 400Mg Tablets
- Ethambutol 400 Mg Tablets
- Ethionamide 250 Mg Tablets
- Metoclopramide 5Mg Paracetamol 500Mg Tablets
- Metronidazole Chewable 250mg Tablets
- Secnidazole Film Coated 500mg Tablets
- Serratiopeptidase 10mg Diclofenac 50mg Tablets
- Pantoprazole 40mg Itopride 150mg (Sr Pellets) Capsules
- Valsartan 40mg Tablets
- Amlodipine 10 Mg Tablets
- Benidipine Hydrochloride 4Mg Tablets
- Azithromycin 250 Mg Tablets
- Acarbose Tablets 25 Mg
- Acyclovir Dispersible Tablets
- Active Pharmaceuticals Ingredients (API)
- Gallery
- Contact Us

Showroom
Active Pharmaceuticals Ingrediants (API) are used in a finished pharmaceutical product, intended to furnish pharmacological activity or to otherwise have direct effect in the diagnosis, cure, mitigation, treatment or prevention of disease. They are the part of any drug that produces the intended effects.
Pharma Intermediates are manufactured using optimum grade raw materials and recent technology under the supervision of our dedicated and experienced experts. These intermediates are the drugs used as raw materials for the production of bulk drugs, or they can refer to a material produced during synthesis of an API.
Pharmaceutical Formulation is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product. The word formulation is often used in a way that includes dosage form. This is defined as the process in which different chemical substances are combined to produce a final medicinal product.
Contact Details
NIKSAN PHARMACEUTICAL
Ankleshwar, Gujarat, India
Ankleshwar, Gujarat, India
- Plot No. 4706/03, Gidc Estate, SF-12, Shrinathji Arcade, Near Meghmani Chowkadi,Ankleshwar - 393002, Gujarat, India
- Email : niksanpharmaceutical@yahoo.com
- Mr SANJAY PATEL (SALES DEPARTMENT)
- Mobile :+919537871777
- niksanpharmaceutical@yahoo.com
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese