NEBIVOLOL
Product Details:
- Storage Room Temperature
- Molecular Weight 405.43 Grams (g)
- HS Code 29420090
- Molecular Formula C22H25F2NO4
- Shelf Life 3 Years
- Medicine Name NEBIVOLOL
- Chemical Name NEBIVOLOL
- Click to View more
NEBIVOLOL Price And Quantity
- 100 Kilograms
NEBIVOLOL Product Specifications
- White crystalline powder
- Other
- 3 Years
- Nebivolol comes in the beta blocker category of medicine. Usually Nebivolol used to treat the high blood pressure. Nebivolol decrease the blood pressure by slowing the heart rate and by relaxing the muscles and blood vessels by this Nebivolol improve the blood flow. Usually Nebivolol is not the first choice medication but it is given when necessary.
- NEBIVOLOL
- NEBIVOLOL
- Room Temperature
- 405.43 Grams (g)
- 118457-14-0
- 29420090
- C22H25F2NO4
- Powder
- 99.8 %
- Medicine Grade
NEBIVOLOL Trade Information
- SAHAR AIR CARGO
- 100 Kilograms Per Month
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Australia North America South America Eastern Europe Western Europe Middle East Africa Central America Asia
- Pondicherry Uttarakhand Dadra and Nagar Haveli Lakshadweep Karnataka Chandigarh Andhra Pradesh Telangana Andaman and Nicobar Islands Jammu and Kashmir Manipur West India Tripura Gujarat Punjab Himachal Pradesh Haryana Rajasthan Kerala Bihar Delhi North India Central India Arunachal Pradesh Goa Sikkim East India Assam Maharashtra Nagaland West Bengal Daman and Diu Meghalaya South India Madhya Pradesh Jharkhand Mizoram Uttar Pradesh Tamil Nadu Odisha Chhattisgarh All India
- FDCA, GMP, GLP AND ISO
Product Description
Niksan Pharmaceutical is worlds top leading manufacturer,exporter, trader and supplier of NebivololAPI as well as finished pharmaceutical products of Nebivolol among the pharmaceutical companies. Our product Nebivolol is widely used and appreciated by our group companies and also our customers and users all around the nations. We offer the Nebivolol in very affordable price.
Niksan Pharmaceutical and Niksan group companies are the very well-known manufacturer, supplier and distributor of Nebivolol products.
Niksan Pharmaceutical are manufacturing and exporting very large quantity of Nebivolol in countries like Hungary, El Salvador, Paraguay, Germany, Argentina, Austria,Romania, Pakistan, Portugal, Latvia, Philippines, Honduras, Switzerland,France, Chile, Estonia, North Macedonia, Lithuania, Guatemala, Croatia, Kenya, Ireland, Serbia, Belgium, Bulgaria, Bosnia & Herzegovina, Netherlands,Colombia, Dominican Republic, Bangladesh, Vietnam, Venezuela, Mexico, Brazil,Iraq, Bolivia, Ecuador, United Arab Emirates, Sri Lanka, Czechia, Australia,Jordan, Spain, Singapore, United Kingdom, Egypt, Slovakia, Greece, Peru,Nigeria, United States, Turkey, Malaysia, Poland, Algeria, Morocco, Hong Kong,Saudi Arabia, South Korea, Denmark, Taiwan, Finland, Thailand, Canada,Indonesia, Italy, Russia and many other countries.
Nebivolol comes in the beta blocker category of medicine. Usually Nebivolol used to treat the high blood pressure.
Nebivolol decrease the blood pressure by slowing the heart rate and by relaxing the muscles and blood vessels by this Nebivolol improve the blood flow. Usually Nebivolol is not the first choice medication but it is given when necessary.
SYNONYMS OF NEBIVOLOL: Narbivolol, Nebivolol, Nebivololum.
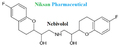
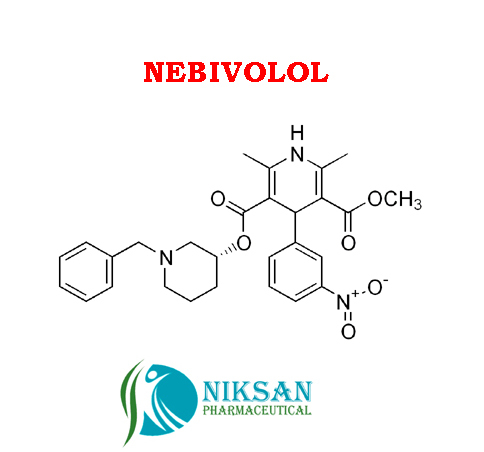
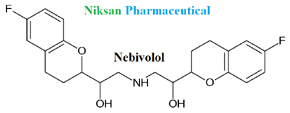
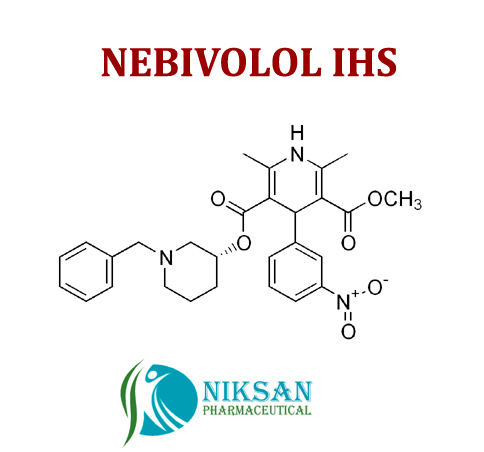
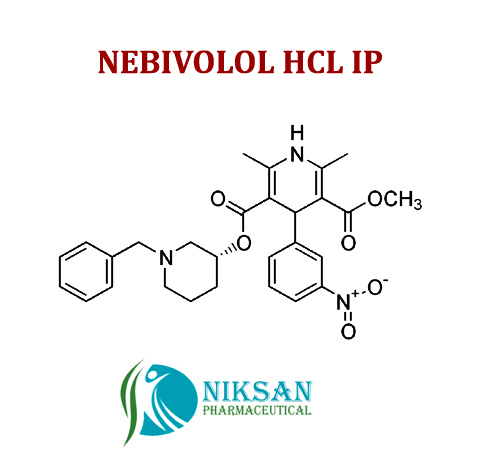
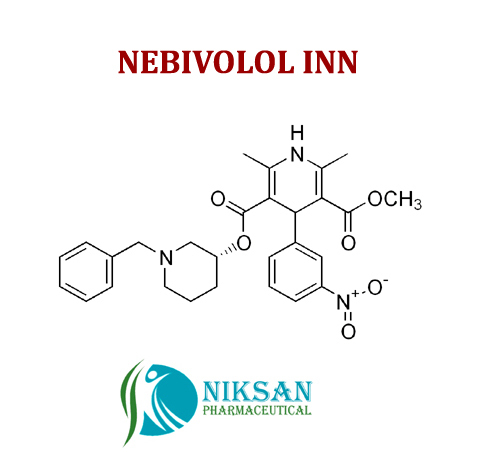
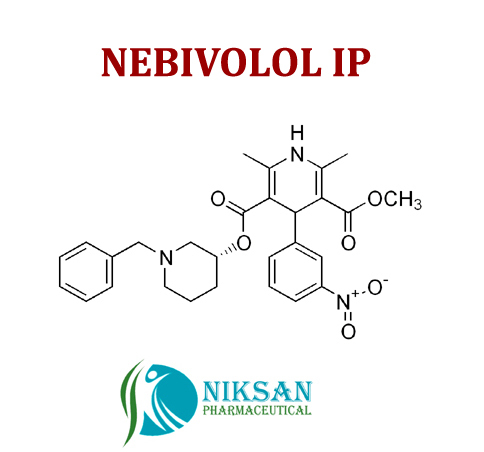
IUPAC NAME OF NEBIVOLOL: 1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)-2-{[2-(6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-yl)-2-hydroxyethyl]amino} ethan-1-ol
CAS NO: 118457-14-0
FORMULA: C22H25F2NO4
MOLECULAR MASS: 405.43g/mol
STORAGE CONDITION: Store in room temperature in dry place, away from light and moisture. Do not put in fridge, kitchen or bathroom.Keep away from children and pets.
HOW TO USE: Take this medication one time per day orally with or without food. Take medication regularly to get more benefits of it, do not forget to take medication, take medication as soon you remember.
HOW NEBIVOLOL WORKS: Nebivolol is normally beta-1 adrenergic receptor antagonist which increases the blood flow and cardiac output by relaxing the blood vessels in the body. The effect of Nebivolol normally stays for 48 hrs.
PHARMACOKINETICS OF NEBIVOLOL: Normally Nebivolol absorbed orally and bind with the 98% of albumin. Nebivolol inhibits the btea-1-adrenargic receptor by this the blood flow increases in heart and body. The half-life of Nebivolol is generally 12hrs. Nebivolol normally excreted 38% by urination and 44% by faces
SIDE EFFECTS OF NEBIVOLOL: The normal side effects like dizziness, headache, weakness, sloe heartbeat, nausea, insomnia are often seen in the patients. Nebivolol sometime cause reduces the blood flow to the feet so feet cold happens some time. If you see some serious side effects like blue figures/toes, fainting, slow heartbeats, mental/ mood swings.
PRECAUTIONS WHILE TAKING NEBIVOLOL: Before taking the Nebivolol tell your doctor if you are allergic to it. Tell your doctor if you have any problem like heart problem, blood circulation problem, breathing problem, liver/kidney problem. Kindly avoid taking medicines if you are in pregnancy period. Before taking the medication tell your doctor if you taking the other medication or having past disease. If the side effects seen worsen kindly contact to your doctor.
CDSCO APPROVAL: Nebivolol + Hydrochlorthiazide approved by CDSCO in India in 23.12.2004,
Indapamide (SR) 1.5mg/1.5mg +Nebivolol 2.5mg/5mg tablet approved by CDSCO in India in 12.05.2010,
Amlodipine (5mg) + Nebivolol (5mg) tablet (addl. Strength) approved by CDSCO in India in 06.09.2007,
Nebivolol 5mg + S(-) Amlodipine 2.5mg Tablet approved by CDSCO in India in 14.09.2004,
Nebivolol (as HCl) 5mg + Valsartan 80mg Capsule approved by CDSCO in India in 25.01.2006,
Nebivolol tabs & bulk import approved by CDSCO in India in 12.07.2002.
FORMULATIONS AVAILABLE IN MARKET:
Nebivolol 5mg tablets
Nebivolol 10mg tablets
Nebivolol 2.5mg tablets
Nebivolol 5mg + Amlodipine 5mg tablets
Nebivolol 5mg + Cilnidipine 10mg tablets
Nebivolol 5mg +Valsartan 80mgtablets
Nebivolol 5mg + Telmisartan 40 mgtablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese