OLMESARTAN
Product Details:
- Smell No Smell
- HS Code 29420090
- Shelf Life 3 Years
- Storage Dry Place
- Molecular Formula C24H26N6O3
- Molecular Weight 446.50 g/mol Grams (g)
- Melting Point 180-182C
- Click to View more
OLMESARTAN Price And Quantity
- 1 Kilograms
OLMESARTAN Product Specifications
- Dry Place
- 446.50 g/mol Grams (g)
- C24H26N6O3
- White to Off-White Solid
- 3 Years
- 99 %
- Pharmaceutical Intermediates
- 29420090
- No Smell
- Odorless
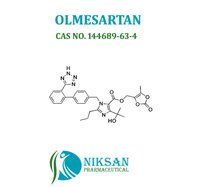
- OLMESARTAN
- 804.2 C
- 180-182C
- Powder
- Medicine Grade
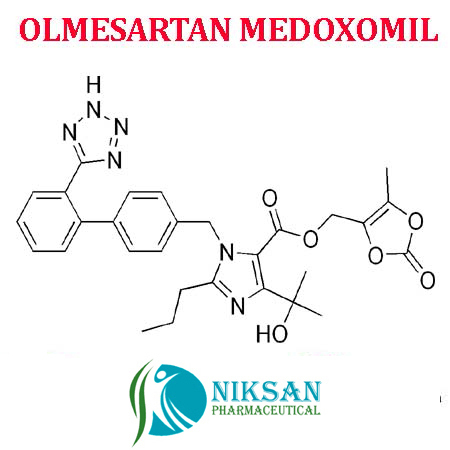
- OLMESARTAN MEDOXOMIL
- Olmesartan belongs to the medicine class called angiotensin receptor blockers. Olmesartan used in the treatment of hypertension. Olmesartan decrease the high blood pressure by relaxing the smooth muscle by vasodilation.
- 144689-24-7
OLMESARTAN Trade Information
- SAHAR AIR CARGO
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Month
- 1 Week
- Yes
- Free samples are available
- HDPE DRUM WITH TWO INNER LDPE LINNER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- Dadra and Nagar Haveli Chandigarh Himachal Pradesh South India North India East India Assam Arunachal Pradesh Chhattisgarh Delhi Gujarat Goa Haryana Jammu and Kashmir Jharkhand Karnataka Madhya Pradesh Maharashtra Mizoram Punjab Rajasthan Tamil Nadu Telangana Tripura West Bengal Nagaland Uttar Pradesh Kerala Sikkim Manipur Meghalaya Andaman and Nicobar Islands Pondicherry Uttarakhand Daman and Diu Lakshadweep Bihar Andhra Pradesh Central India Odisha West India All India
- FDCA, GMP, GLP AND ISO 9001-2014
Product Description
Niksan Pharmaceutical is one of the leading manufacturer, supplier and exporter of the Olmesartan. The best productmanufactured by Niksan Pharmaceutical is Olmesartan. We manufacture, supply and export best quality product of Olmesartan API along with Olmesartan formulations.
Niksan Pharmaceutical provides best quality product of Olmesartan in all states of India like Kerala, Punjab, Rajasthan, Telangana, UttarPradesh, Gujarat, Delhi, Tamil Nadu, Bihar, Karnataka, Jammu and Kashmir,Maharashtra, West Bengal, Odisha, Madhya Pradesh, Andhra Pradesh, Haryana and many other states.
Niksan Pharmaceutical is also manufacture and exports large quantity of Olmesartan products in whole globe since many years in countries like Guatemala,Venezuela, Honduras, Bangladesh, Nicaragua, Ireland, El Salvador, Italy, United States , Australia, Dominican Republic, Mexico, Portugal, Canada, Chile, South Korea, Spain, United Arab Emirates, Philippines and many more countries.
Olmesartan belongs to the medicine class called angiotensin receptor blockers. Olmesartan used in the treatment of hypertensi on. Olmesartan decrease the hi gh blood pressure by relaxing the smooth muscle by vasodilation.
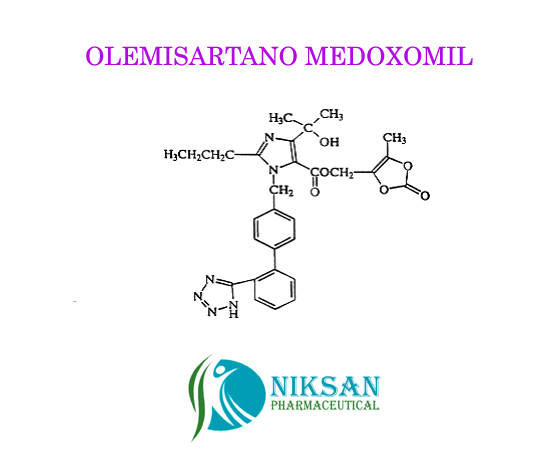
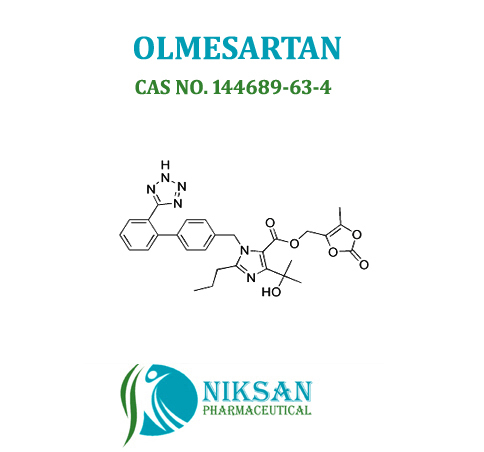
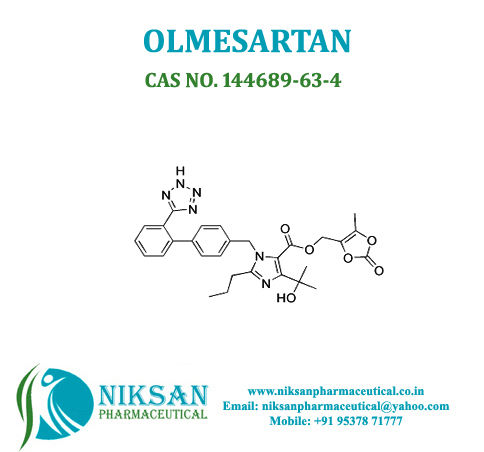
IUPAC NAME: 4-(2-hydroxypropan-2-yl)-2-propyl-1-({4-[2-(1H-1,2,3,4-tetrazol-5-yl) phenyl] phenyl} methyl)-1H-imidazole-5-carboxylic acid
CAS NO: 144689-24-7
FORMULA: C24H26N6O3
MOLECULAR MASS: 446.50g/mol
STORAGE CONDITIONS: Store medicine in cool and dry place, away from direct heat and sunlight. Do not store medicine in humid place like bathroom or kitchen. Keep away from children and pets.
HOW TO USE: Take medicine once per day by mouth. If you are using the oral liquid form, shake the container before use. Carefully measure the liquid dosage form.
HOW OLMESARTAN WORKS: Olmesartan inhibits the high blood pressure by binding with angiotens in II receptor . Angiotensin II cause vasoconstriction so by inhibiting the enzyme Olmesartan cause vasodilation of the smooth muscle and by this blood flow increases and prevents hypertension.
PHARMACOKINETICS: Olmesartan rapidly absorbed IN GI track after the oral administration with the bioavaibility of 4.5% to 28.6%. Almost 99% of Olmesartan binds with the blood plasma albumin. The half-life of Olmesartan is 10-15 hours. Approximately 10-16% of Olmesartan eliminated through urine.
SIDE EFFECTS: Common side effects of Olmesartan are lightheadness and dizziness. Other side effects like fainting, high potassium blood level, signs of kidney problem. Some other rare reactions like skin rash,itching, swelling of skin, dizziness, trouble in breathing.
PRECAUTIONS: Before talking the Olmesartan medicine,tell your doctor if you are allergic to Olmesartan. Tell your doctor about your medical history like liver disease, kidney disease, dehydration or any respiratory problem. This medicine makes you dizzy so kindly avoid taking alcohol and cannabis products. Also avoid driving or operating machineries because Olmesartan makes you dizzy.
CDSCO APPROVAL: FDC of Olmesartan Medoxomil (20mg/40mg/40mg) + Hydrochlorthiazide(12.5mg/12.5mg/25mg)approved by CDSCO in India in 02.12.2005,
Amlodipine Besilate IP eq.to Amlodipine 10mg/5mg/5mg/5mg/2.5mg +Olmesartan Medoxomil 40mg/40mg/20mg/5mg/5mg + Hydrochlorothiazide IP25mg/12.5mg/12.5mg/12.5mg/12.5mg Tablet 23.11.2011
Olmesartan Medoxomil (20mg) + Amlodipine Besylate (5mg) Tablet approved by CDSCO in India in 15.10.2007,
Metoprolol succinate ER 25mg+50mg + Olmesartan 20/20mg approved by CDSCO in India in 19.07.2010,
Olmesartan 40mg + Amlodipine 5mg (Additional Strength) approved by CDSCO in India in 09.08.2010,
Olmesartan medoximil-20mg + Ramipril-5mg. Tablet approved by CDSCO in India in 12.6.2007,
Olmesartan (20/40mg) + Atorvastatin 5/10/20mg film coated tablets approved by CDSCO in India in 27.10.2009,Olmesartan + Ramipril (40mg + 5mg) tablets (Additional Strength)approved by CDSCO in India in 15.09.2008,
Olmesartan (as Medoxomil) 10mg (addl. Strength) approved by CDSCO in India in 18.05.2006,
Olmesartan Medoxomil (5mg/20mg/40mg) Tablet approved by CDSCO in India in 21.07.2005,
Olmesartan 40mg + Hydrochlorthiazide 25mg tablet (addl. Strength)approved by CDSCO in India in 28.06.2006.
FORMULATIONS AVAILABLE IN MARKET:
Olmesartan Medoxomil 5mg tabl ets
Olmesartan Medoxomil20mg tablets
Olmesartan Medoxomil 40mg tablets
Olmesartan Medoxomil 10mg tablets
Olmesartan Medoxomil 20mg + amlodipine 5mg tablets
Metoprolol succinate ER 25mg+ Olmesartan 20mg tablets
Metoprolol succinate ER 50mg + Olmesartan 20mg tablets
Olmesartan + Ramipril (40mg + 5mg) tablets
Olmesartan 40mg + Hydrochlorthiazide 25mg tablets
Olmesartan 40mg + Amlodipine 5mg tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese