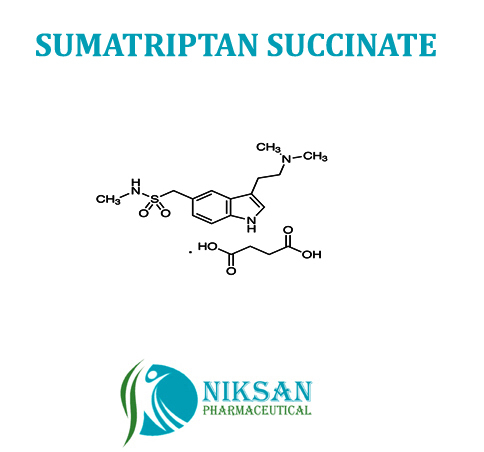

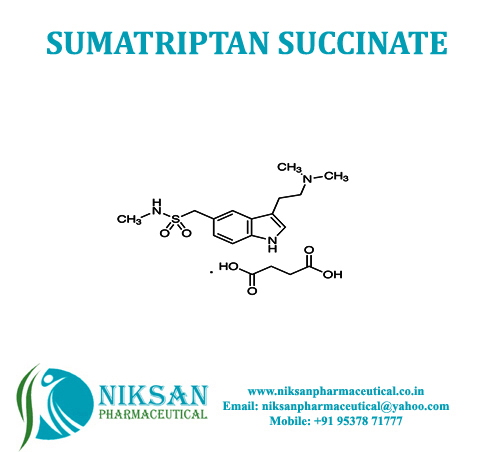
SUMATRIPTAN
Product Details:
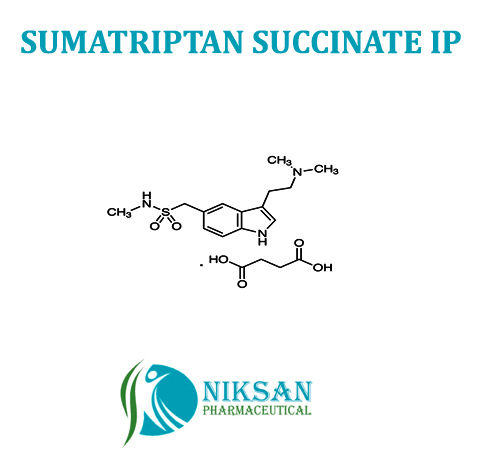
- Molecular Formula C14H21N3O2S
- Shelf Life 3 Years
- Storage Room Temperature
- HS Code 29420090
- Molecular Weight 295.4 g/mol GSM (gm/2)
- Medicine Name SUMATRIPTAN
- Chemical Name SUMATRIPTAN
- Click to View more
SUMATRIPTAN Price And Quantity
- 5 Kilograms
SUMATRIPTAN Product Specifications
- Other
- 295.4 g/mol GSM (gm/2)
- Medicine Grade
- 103628-46-2
- SUMATRIPTAN
- 29420090
- SUMATRIPTAN
- White to Off-White Solid
- Room Temperature
- 3 Years
- C14H21N3O2S
- 99 %
- Powder
- Sumatriptan is belongs to drugs class called selective serotonin receptor agonists which is used to treatment of migraine and cluster headaches.
SUMATRIPTAN Trade Information
- NHAVA SHEVA
- Paypal Cash Against Delivery (CAD) Cash on Delivery (COD) Cash Advance (CA) Cash in Advance (CID) Cheque Days after Acceptance (DA) Delivery Point (DP) Letter of Credit at Sight (Sight L/C) Telegraphic Transfer (T/T) Western Union Letter of Credit (L/C)
- 100 Kilograms Per Week
- 1 Days
- Yes
- Free samples are available
- HDPE DRUM WITH TWO LDPE INNER LINER
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- All India South India Central India West India North India East India Gujarat Karnataka Kerala Lakshadweep Mizoram Meghalaya Manipur Andhra Pradesh Bihar Chandigarh Daman and Diu Goa Jharkhand Odisha Punjab Assam Delhi Dadra and Nagar Haveli Andaman and Nicobar Islands Arunachal Pradesh Chhattisgarh Haryana Himachal Pradesh Jammu and Kashmir Madhya Pradesh Maharashtra Nagaland Rajasthan Sikkim Tamil Nadu Telangana Tripura Pondicherry Uttar Pradesh Uttarakhand West Bengal
- WHO GMP,GMP,GLP,ISO
Product Description
Niksan Pharmaceutical is top leading manufacturer, supplier, exporter and trader of Sumatriptan in Indian states and also in world other countries. Sumatriptan is the best product of Niksan Pharmaceutical which exporting as a form of API and finished formulations.
Our product Sumatriptan is world widely appreciated by our group companies and clients. We give best possible selling price of Sumatriptan to our clients, because customer satisfaction is our first priority.
Niksan Pharmaceutical and Niksan group companies are the largest manufacturer, supplier and distributor of Sumatriptan API as well as Sumatriptan finished formulations provide in many Indian states like Kerala, AndhraPradesh, Tamilnadu, Telangana, Karnataka, Punjab, Gujarat, Maharashtra, Delhi,Haryana, West Bengal, and Rajasthan, UP, Jammu and Kashmir and many others Indian states for many years.
Niksan Pharmaceuticals are the largest exporter, supplier, and manufacturer of the Sumatriptan API and Sumatriptan finished formulations in many countries since many years like Nepal, UAE, Bangladesh, South Korea,Philippines, Australia, United Kingdom, United States, Estonia, Eswatini, Ethiopia,Fiji, Finland, France, Gabon, Gambia, Georgia, Liberia, Libya, Liechtenstein,Lithuania, Luxembourg, Malawi, Malaysia, Maldives, Mali, New Zealand,Nicaragua, Pakistan, Palau, Panama, South Africa, South Sudan, Spain and many other countries.
Sumatriptan is belongs to drugs class called selective serotonin receptor agonists which is used to treatment of migraine and cluster headaches.
SYNONYMS: Sumatriptan,Sumatriptanum
IUPAC NAME: 1-{3-[2-(dimethyl amino) ethyl]-1H-indol-5-yl}-N-methylmethanesulfonamide
CAS NO: 103628-46-2
FORMULA: C14H21N3O2S
MOLECULAR MASS: 295.4 g/mol
STORAGE OF SUMATRIPTAN: Keep Sumatriptan medicine in air tight container. Keep away from excess heat and moisture. Store it at room temperature.Keep all medication out of sight and reach of children. Do not store in bathroom and do not flush this medicine.
APPLICATIONS OF SUMATRIPTAN: Sumatriptan used as a pain killer which helps to relive headache and pain. Sumatriptan is also use to treatment of migraine symptoms like vomiting, nausea and sensitivity to light or sound.
HOW TO USE: Sumatriptan should take by mouth with or without food. Follow all directions on your prescription label or as your doctor recommends you. You can take Sumatriptan at first sign of a migraine headache. If the condition does not improve after taking Sumatriptan, you can take another tablet of Sumatriptan 2 hours after the 1st dose.
HOW SUMATRIPTAN WORKS: It constricts the blood vessels in the head and reducing the signals of pain sent to the brain. Sumatriptan work on blood vessel in your brain and control stimulating serotonin receptors in the brain.
CONTRAINDICATIONS: In your past history you have a heart attack or serotonin syndrome kindly avoid Sumatriptan medicine. Do not take this medicine if you have a problem of chest pain or Prinzmental angina. Kindly avoid Sumatriptan if you are a patient of high blood pressure and low supply of oxygen. Discuss with your doctor if you are an allergic to Sumatriptan.
PHARMACOKINETICS OF SUMATRIPTAN: Absorption of Sumatriptan inhuman body is weak after oral administration.Half-life of Sumatriptan is 2 hours as clinical pharmacology.Peak plasma concentration of Sumatriptan is 75% and usually reached within 45 min.Approximately 40% Sumatriptan excreted as feces and 22% excreted by urine.
SIDE EFFECTS OF SUMATRIPTAN:Flushing,feelings of tingling, numbness, prickling, heat, tiredness, weakness,drowsiness, or dizziness are most common side effects are seen in patient.Sometimes this medicine may raise your blood pressure so check your blood pressure regularly during the dose of Sumatriptan.Sumatriptan cause bloody diarrhoea, trouble in speaking, sign of a stroke, shortness of breath.A very serious side effect to this drug is rare but get help your doctor if you are noticed any serious symptoms.
PRECAUTIONS:Check with your doctor if you take this medicine regularly and it did not work.Do not take Sumatriptan if you have used an MAO inhibitor.Discuss with your doctor before taking this medicine if you are a patient of heart dieses.Sumatriptan makes you dizzy so kindly avoid alcohol and do not drive vehicle.
CDSCO APPROVAL: Sumatriptan 85 mg + Naproxen 500 mg Tablets are approved by CDSCO in India in 03.08.2009
Sumatriptantablets are approved by CDSCO in India in 06.02.1996
Sumatriptan+ Naproxn (50mg + 275mg) Tablets are approved by CDSCO in India in 24.11.2008
Sumatriptan nasal sprayare approved by CDSCO in India in 29.08.2001
FORMULATIONS AVAILABLE IN MARKET:
Sumatriptan tablets
Sumatriptan 50 MG tablets
Sumatriptan 25 MG tablets
Sumatriptan 100 MG tablets
Sumatriptan 20 MG tablets
Sumatriptan 6 MG tablets
Sumatriptan nasal spray
Naproxen 550MG +Sumatriptan 50 MG tablets
Sumatriptan+ Naproxen (50mg + 275mg) Tablets
Sumatriptan85 mg + Naproxen 500 mg Tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are stillvalid and its liability is at Buyers Risk.
REFERENCES:
www.webmd.com
https://pubchem.ncbi.nlm.nih.gov
https://go.drugbank.com
https://cdscoonline.gov.in
https://www.wikipedia.org/
https://www.drugs.com
https://www.zaubacorp.com
https://www.practo.com/consult
Other Products in 'Active Pharmaceuticals Ingredients (API)' category
 |
NIKSAN PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese